Here are the essential concepts you must grasp in order to answer the question correctly.
Polarity of Water
Water is a polar molecule, meaning it has a partial positive charge on one side (hydrogen atoms) and a partial negative charge on the other side (oxygen atom). This polarity allows water molecules to interact with each other and with other substances, leading to unique properties such as cohesion and adhesion.
Recommended video:
Hydrogen Bonding
Hydrogen bonds are weak attractions that occur between the positively charged hydrogen atoms of one water molecule and the negatively charged oxygen atoms of another. These bonds are responsible for many of water's unique properties, including its high surface tension, boiling point, and ability to dissolve many substances.
Recommended video:
Molecular Motion
Water molecules are in constant motion due to thermal energy, which affects their interactions. This motion contributes to the dynamic nature of water, influencing its state (solid, liquid, gas) and its behavior in various environments, such as its ability to expand upon freezing.
Recommended video:
Pacemakers: Molecular Physiology
 Verified step by step guidance
Verified step by step guidance Verified Solution
Verified Solution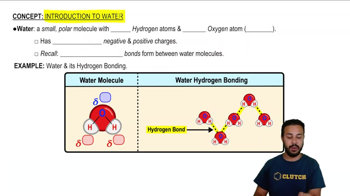



 5:19m
5:19m