Here are the essential concepts you must grasp in order to answer the question correctly.
Functional Groups
Functional groups are specific groups of atoms within molecules that are responsible for the characteristic chemical reactions of those molecules. They determine the properties of organic compounds, including their polarity and solubility. Common functional groups include hydroxyl, carbonyl, and amino groups, each influencing the behavior of the compound in biological and chemical contexts.
Recommended video:
Polarity
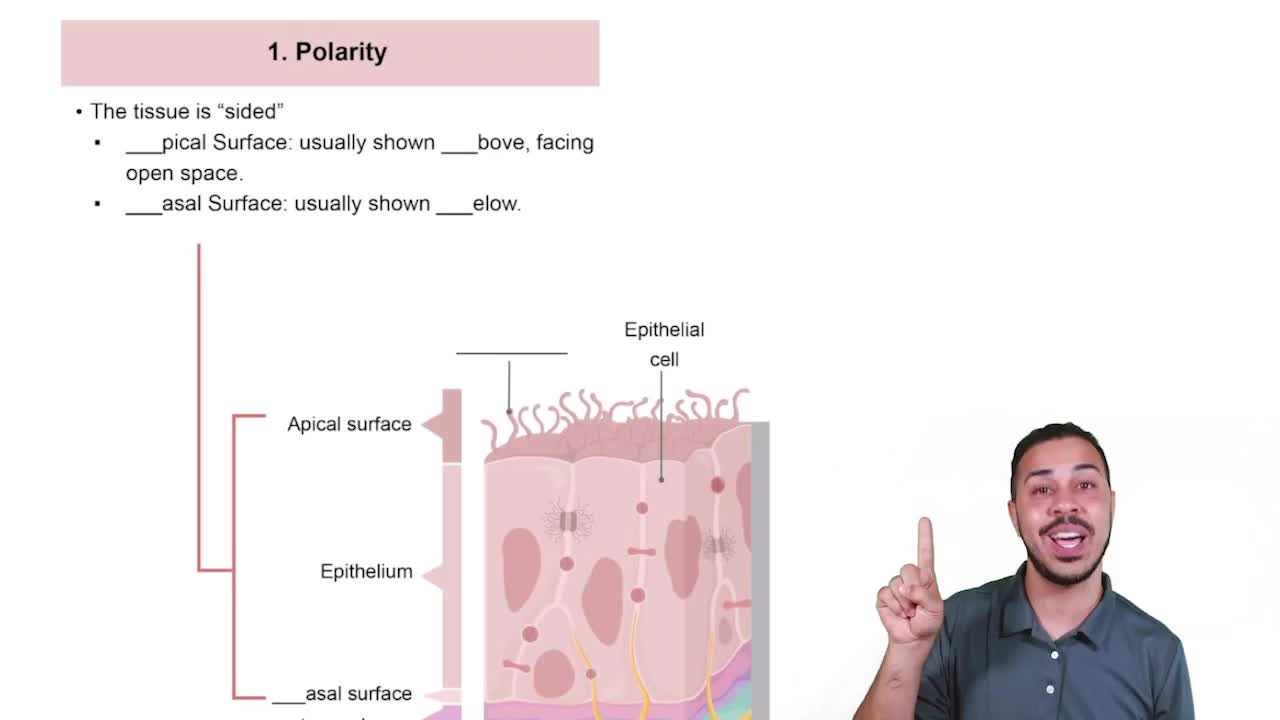
Polarity refers to the distribution of electrical charge over the atoms in a molecule. A polar molecule has a partial positive charge on one side and a partial negative charge on the other, leading to an uneven distribution of charge. This property affects how molecules interact with each other, particularly in terms of solubility in water, where polar molecules tend to be hydrophilic (water-attracting).
Recommended video:
Characteristic 1: Polarity
Hydrophilicity
Hydrophilicity is the property of a substance to interact favorably with water, often due to the presence of polar functional groups. Compounds that are hydrophilic can dissolve in water, making them essential in biological systems where water is a solvent. Functional groups like hydroxyl (-OH), amino (-NH2), and carbonyl (C=O) contribute to this property, enhancing the solubility of organic compounds.
Recommended video:
Hydrophilic vs. Hydrophobic