Multiple Choice
Which of the following effects can occur because of the high surface tension of water?
2873
views
32
rank
 Verified step by step guidance
Verified step by step guidance Verified Solution
Verified Solution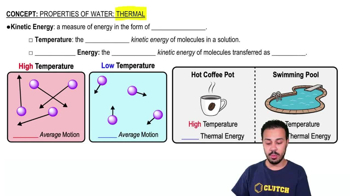

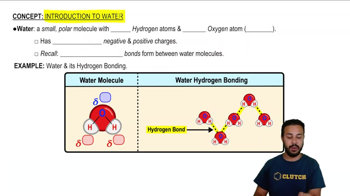

 3:52m
3:52mMaster Properties of Water- Cohesion and Adhesion with a bite sized video explanation from Jason Amores Sumpter
Start learning