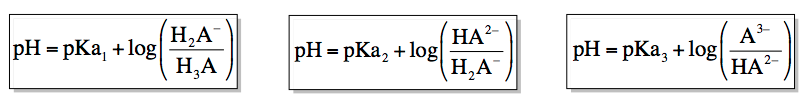Polyprotic buffers, often synonymous with triprotic buffers, are characterized by their ability to donate three acidic hydrogens. A triprotic acid has three distinct acid dissociation constants, denoted as pKa values, which correspond to the sequential removal of these hydrogens. The fully protonated form of a triprotic acid is represented as H3A, while the removal of each hydrogen leads to intermediate forms: H2A- after the first dissociation (Ka1), HA2- after the second (Ka2), and A3- after the third (Ka3).
In addition to the acid dissociation constants, the base dissociation constants (Kb1, Kb2, Kb3) can be considered when adding back acidic hydrogens. Understanding the relationships between these constants is crucial for working with polyprotic acids. The Henderson-Hasselbalch equation can be adapted for each form of the polyprotic buffer, allowing for the calculation of pH based on the concentrations of the acid and its conjugate base.
The first form of the Henderson-Hasselbalch equation relates the pH to the first dissociation constant:
pH = pKa1 + log([Intermediate 1] / [Acid])
In this equation, the more acidic form (with more H+ ions) is placed in the denominator. The second form, which involves the second dissociation constant, is expressed as:
pH = pKa2 + log([Intermediate 2] / [Intermediate 1])
Finally, the third form relates to the transition from the second intermediate to the basic form:
pH = pKa3 + log([Basic Form] / [Intermediate 2])
Citric acid serves as a common example of a triprotic acid, possessing three acidic hydrogens. When calculating pH using the Henderson-Hasselbalch equation, one can use either molarity or moles. For instance, if you have 0.10 M citric acid and 0.15 M of the first intermediate form, the pH can be calculated as:
pH = -log(7.4 × 10-4) + log(0.15 / 0.10) = 3.31
In another scenario, using moles instead of molarity, the pH can be calculated similarly, ensuring that the more basic form is always placed in the numerator. For example:
pH = -log(1.7 × 10-5) + log(0.25 / 0.10) = 4.94
Lastly, when dealing with volumes and molarities, it is essential to convert milliliters to liters and calculate moles accordingly. For example:
pH = -log(10-7) + log(0.016 / 0.015) = 6.43
In summary, understanding the specific forms of a polyprotic buffer is critical for determining which Ka value to use in the Henderson-Hasselbalch equation. This knowledge is fundamental as one navigates through the complexities of buffer systems.