So here we're told that lanthanum(III) hydroxide has a Ksp of 2.0 times 10 to the negative 21. It says, how many grams of lanthanum(III) hydroxide are dissolved as hydroxide ions in 2.5 liters of a saturated solution of lanthanum(III) hydroxide. Alright. So we're dealing with the Ksp of this compound. If we're dealing with Ksp, that means we're dealing with the solid form of this compound and we look to see how it dissolves in water.
When it dissolves in water, it establishes an equilibrium. So we're going to have 1 lanthanum(III) ion. Ions when dissolved in water are aqueous plus 3 hydroxide ions. Here, we're going to set up an ICE chart when dealing with Ksp. So we have initial, change, equilibrium.
With an ICE chart, we ignore solids and liquids. So here, my solid will be ignored. Products are being formed over time, so this will be 0 + x. Because this is a 3, this is plus 3x, plus x, plus x plus 3x.
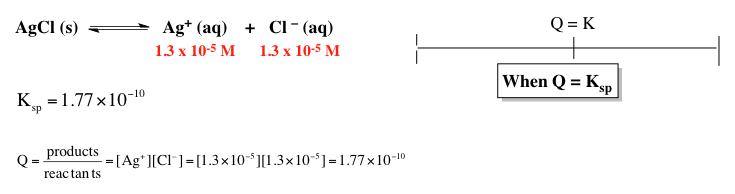
Now we're going to say Ksp, which is your solubility product constant equals products over reactants just like all of the equilibrium constants. But here for this equation, our reactant is a solid so we're going to ignore it. So Ksp here will just equal products. So it equals the concentration of lanthanum ion times hydroxide ion concentration because, again, this coefficient is 3.
Now this is going to be to the 3rd power. So that coefficient makes it 3x, but also makes it raised to that power. Ksp, again, is 2.0 times 10 to the negative 21. At equilibrium, La3+ is x, OH- is 3x. So that's x times 27, and then that's x cubed.
27xx4=2.0×10-21
So here, we need to isolate x here. Divide both sides here by 27. When we do that here, that's going to give us 7.40741×10-23=x4. Now we need to just isolate x, not x to the 4th.
So I'm going to take the 4th root of both sides. For those of you who don't have a button for the 4th root, you may have to use the power function to calculate x to the 1/4. So that will give us x equals 2.934 times 10 to the negative 6 molar.
Now, regarding the original question, it's asking us to find grams. How many grams of lanthanum(III) hydroxide are dissolved as hydroxide ions?
We're looking for grams of hydroxide ions. The wording is a little tricky, but that's what we're looking for. If we need to find the grams of hydroxide ions, we need to first figure out its concentration. At equilibrium, hydroxide ion concentration equals 3x. So that's 3 times the answer we just got. So OH- concentration here equals 8.80 times 10 to the negative 6 molar.
Remember that moles equals liters times molarity. We're told that we have 2.5 liters, so multiply that by the molarity we just isolated for hydroxide ions and that'll give us the moles of hydroxide ions, which comes out to 2.20 times 10 to the negative 5 moles of hydroxide ions.
All we have to do now is just change those moles into grams. So one mole of hydroxide ions consists of 1 oxygen and 1 hydrogen. So looking at your periodic table, the masses are 15.99994 grams and 1.00794 grams. Add them together gives us 17.00784 grams, roughly, of hydroxide ions.
Moles cancel out, and we'll get grams at the end which comes out to 3.74 times 10 to the minus 4 grams of hydroxide ion. So as you can see, there's a great deal of work that's required in terms of answering this question. Read the question very carefully. Here, we're not looking for grams of the entire ionic compound. We're looking for grams of lanthanum(III) hydroxide in the form of hydroxide ions.
So we're actually looking for the grams of hydroxide ions in this question. Anytime we find x, x gives us the solubility or concentration of our ionic compound as a whole. If we want the concentration of the particular ions, we have to look at the ICE chart. At equilibrium, this ion was just equal to x, so it'd be the same exact number. But for hydroxide ions, at equilibrium, it equals 3x.
So it'd be 3 times what we found for x to get the concentration of hydroxide ion. From there, we can change concentration to moles by using liters, and then change those moles into grams. Now that you've seen this example, move on ahead to example 2. See if you can approach this question. If you can't, don't worry.
Just come back and see how I tackle this example 2 question.