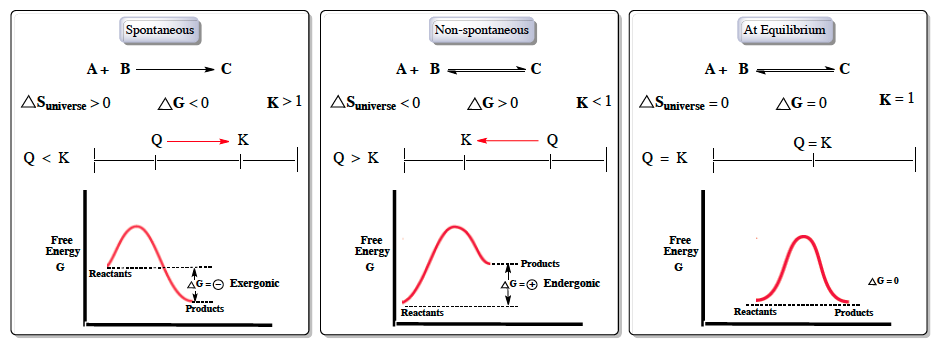
Hey everyone. So when we talk about Gibbs free energy, it's usually in reference to spontaneous reactions. Now, spontaneous reactions can occur without any outside energy being invested; they happen naturally. Non-spontaneous reactions, on the other hand, cannot occur naturally. Now, when we talk about spontaneous reactions, we're talking about spontaneous, non-spontaneous, and being at equilibrium.
And with these ideas, we have the variables of Gibbs free energy, entropy of our universe, which is tied to the second law of thermodynamics, and then our equilibrium constant K. So if we take a look at spontaneous reactions, here we have our reactants A and B producing our product C. For this to be a spontaneous result, we would say that the entropy of our universe would have to be greater than 0, following the second law of thermodynamics. Gibbs free energy Δg would have to be less than 0, and then our equilibrium constant K would have to be greater than 1. Typically, when we're talking about a spontaneous reaction that means that the reactants naturally flow in the forward direction to create products.
We can attach this idea to the whole thing of the reaction quotient q. To move in the forward direction, the reaction quotient q would have to be less than k. Remember that q means the shift to get to equilibrium, to get to k. Since q is less than k, q would move in the forward direction to get to k, which would mean that the reaction would also move in the forward direction. Now, if we're to talk about Gibbs free energy in terms of an energy diagram, realize that we have our reactants and then we're trying to get to our products.
For a reaction to be favorable, we'd want our products to be lower in overall energy. So here we'd see that our reactants are starting up at a higher energy level, and our products are ending at a lower level. Here to understand the value associated with this is just that Δg equals products minus reactants. So this is the equation we can link to each one of these energy diagrams. As a result of products being lower in energy, that means our Δg value overall is negative or what we call exergonic.
For a non-spontaneous reaction, it's basically the opposite process. So signs get flipped; here we'd say that our entropy of the universe would be less than 0, Gibbs free energy would be greater than 0, k would be less than 1. In a non-spontaneous reaction, that means that it's not favorable in the forward direction, but it would be favorable in the reverse direction. So here, to favor the reverse direction, that happens when q, our reaction quotient, is greater than k. So here since q is greater than k, q always shifts to wherever k is, so it's going to shift in the forward reverse direction to obtain equilibrium.
Here, when we're talking about the energy diagram again, this time, products will be higher in energy, which is not something we want. The whole purpose of most chemical reactions is to create a product that is lower in energy. Following products minus reactants, ΔG would be positive here, meaning that it is endergonic. And then finally, when we're neither spontaneous nor non-spontaneous, we are at equilibrium. So here ΔS of Universe would be equal to 0.
We'd say that Gibbs free energy is equal to 0. K is equal to 1. Since we're at equilibrium, our reaction quotient q is equal to our equilibrium constant k, so there's no shifting. And then looking at our energy diagrams, reactant products will end at the same energy level, so overall our Δg value will be equal to 0. Now, beside these conceptual ideas, we can also talk about the formulas associated with our Gibbs free energy.
Gibbs free energy can also be calculated through the use of various equations. So here we have Gibbs free energy under non-standard conditions equals the change in enthalpy minus T times the change in entropy. Here, realize that there's no circle here. That means that when there's no circle here, that's non-standard conditions. If I were to talk about standard conditions, I'd put a little circle there for each of them.
Standard conditions would mean that our pressure is 1 atmosphere. Standard conditions would also mean that our molarity is 1 molar. Standard conditions here could also mean that our temperature is 25 degrees Celsius. Now with our Gibbs free energy under non-standard conditions, we can have it equal to Gibbs free energy under standard conditions plus RT times lnq. R here represents our gas constant and since we're talking about energy it would be in the form of 8.314 joules over moles times k.
T here would be our temperature in Kelvin, and q again is our reaction quotient which is equal to products over reactants. And then finally, we can associate our Gibbs free energy under standard conditions with our equilibrium constant k by the use of this equation negative RT ln k. So, just remember that a spontaneous reaction can occur naturally without the use of outside energy. Associated with this idea of spontaneity, we have variables of Delta S of the universe, our equilibrium constant k, as well as, of course, our Gibbs free energy which is Delta g.