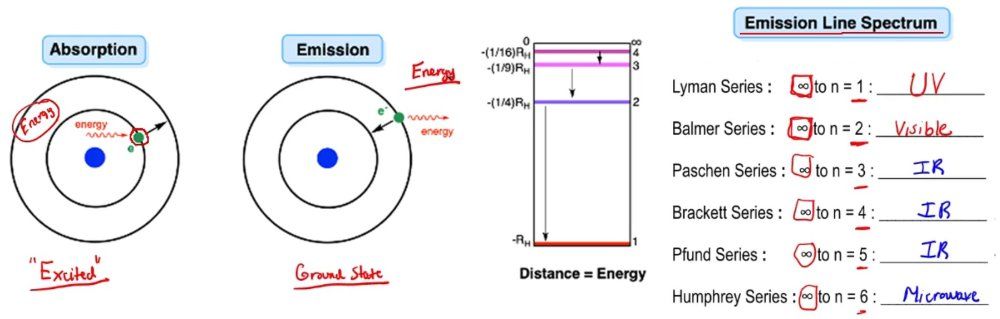
So here we talk about the ideas of emission versus absorption. Now absorption involves the taking in of excess energy by an electron or an atom and promoting itself to a higher energy state. Here, if we're going by Bohr's model of the hydrogen atom, we have our electron here in the first shell of the hydrogen atom. This ambient outside energy is absorbed. It takes in this outside energy and uses it to drive itself up to a higher energy state.
We'd say when it absorbs it gets to an excited state. However, you can't hold on to that excess energy forever and eventually you have to let it go. When it releases that excess energy that it absorbed earlier, it's going to emit that energy and drop back down to its ground state. Now we can talk about the movement of an electron from one shell to another shell and the type of energy involved in either absorption or emission. Now we're going to say if we're talking about emissions, we could talk about emission line spectrums.
Here they each have different names. We can say that the number of shells were at the moment we can go up to 7 based on the periodic table. There are 7 rows on the periodic table, so we can talk about elements having 7 shells. But remember, the periodic table is not static. It's dynamic.
As we discover new elements, as we explore space, maybe when we get to Mars, we find something new, or maybe we come up with more advanced technology that allows us to create more synthetic elements in the lab, the number of shells can greatly expand beyond 7. Maybe in 100 years there will be an additional row or 2. That's the way the periodic table works. For now we're talking about these particular emission line spectrums, meaning that our electron or our excited atom drops from a higher shell number to its original ground state, and depending on where it lands, it has a specific name to it. So if you're going from a higher shell number, whatever it is, and you're dropping back down to shell number 1, which is your original ground state, we call that a Lyman series.
Now in terms of electromagnetic radiation, a Lyman series would fall within the UV spectrum of our electromagnetic spectrum. The Balmer series, you're going from a higher shell number and you're dropping down to the second shell. We're going to say here, you're going to basically emit light in the visible light region. This type of emission we can see because it falls within the part of the electromagnetic spectrum that we can see without the aid of any technology. We can use our eyes.
For the Hessian series, we're going from a higher shell number down to shell number 3. This falls within IR, and in fact, the next 2 all fall within IR. Finally, the Humphrey series falls closer to the microwave region, so we're gonna say this one's closer to the microwave region of the electromagnetic spectrum. Remember, distance equals energy. As we can see, if you're going from the second shell down to the first shell, this is the distance you have to traverse.
Going from the 3rd shell down to the 2nd shell, this is the distance. 3rd shell to the 2nd shell, that's the distance. You can see as you go higher up with the shell numbers, the distance between shells gets smaller and smaller. So we can say that in terms of energy, it's increasing going this way because whatever shell this is when we drop down to the first shell that's a big distance to cover. If this isn't 2 and it's one of the even larger numbers, then it's an even greater distance you have to fall.
We can say in terms of this chart that we have our potential energy. We can say our potential energy equals our Rydberg constant, which is negative 1.8 times 10 to the negative 18 joules times 1 over n squared. So you can figure out the potential energy your electron has in each shell. Now, we said that the Balmer series deals with the visible light spectrum in terms of its emission. From this, we have what are called emission spectrums and absorption spectrums.
Now, if we're dealing with, let's say we're dealing with a cloud here. We're going to say here for an emission spectrum it represents the different frequencies of electromagnetic radiation emitted by an atom as it transitions from a higher energy state to a lower energy state. Here we have a cloud of particles and we can say that this cloud of particles has a higher than normal temperature, and we're going to say that it emits certain forms of radiation as the electrons or atoms fall down to a lower energy state. These bits of energy are filtered through this slit and pass through this prism, and from this, we're able to come up with an emission spectrum. In an emission spectrum, we have a black background and on it are projected strips of colors.
By analyzing these stripped colors here, we can identify the element that we're dealing with because different elements produce slightly different emission spectrums. Now what's the difference between that and an absorption spectrum? Well, in an absorption spectrum, things are inverted. Now we have a colored background which represents the visible light spectrum, and then we have these black bands here that we can look at to help us identify our unknown element. Here in this case we have some type of energy source that passes through an atom, a cloud, an electron, and then that is passed through the slit, and then through a prism to help create this absorption spectrum.
So the difference between an emission spectrum and an absorption spectrum is how it looks, but really how the energy is filtered through the slit, then through the prism. Here, the energy comes directly from a cloud of particles or an atom itself that's excited and it gets pushed into the slit and then to the prism to create this emission spectrum. For an absorption spectrum, it's first filtered through an atom. An outside energy source filters through the atom which then filters through the slit to get to the prism to create an absorption spectrum. Just remember fundamentally absorption means you're taking in energy to jump up to a higher energy state.
Emission, you release that excess energy you took in earlier to drop back down to your original ground state level. Remember that with each type of emission, we have a series name involved, and these series names are connected to the electromagnetic spectrum where they fall somewhere between UV and the microwave region. The greater the fall from a higher energy state to a lower energy state, the more energy that's released.