Titrations are an important topic that we're going to discuss when it comes to stoichiometric reactions, but we'll talk about it consistently throughout many of the chapters as we delve deeper and deeper into analytical chemistry. Here, we're going to say that connected to the idea of titrations, we have other terms that you should recall. Here we're going to say that when we're dealing with gravimetric analysis, this is just basically helping us to determine the amount of product that we can isolate within a chemical reaction. We're going to say the calculations that we use to determine that isolated amount is termed stoichiometry. With stoichiometry, we have to employ the use of the stoichiometric chart.
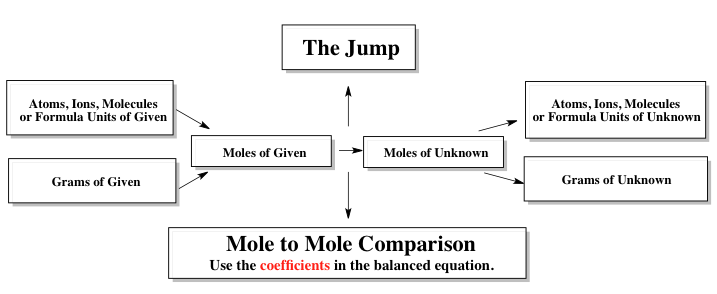
Remember, with the stoometric chart, we'll be given a balanced chemical reaction and from that balanced chemical reaction, we'll be given amounts for either some elements and compounds, and asked to find the unknown quantity of some other element or compound within that balanced equation. Now with the stoichiometric chart, we have our given information which typically is given to us in some units of mass or substance. They may give us given amounts in grams, possibly moles. Remember, when we're going from grams to moles, we use the molecular weight of the compound itself. They may give us the initial amount in atoms, ions, molecules or formula units.
In this case, to go to moles, we'd use Avogadro's number which is 6.022×1023. Remember when we use the term atoms, atoms are basically for neutral single elements. So, if we're talking about oxygen atom, we're just talking about O. We use the term for ions when we have a single element with a charge. Then, the term molecules is used for covalent compounds, which are just nonmetals connected together.
And formula units are used for ionic compounds where we have a positive ion connected to a negative ion. Now, using the stoichiometric chart, we'll be given information. Usually one of these values, and it's our job to go to the unknown amount of our other compound or element within the balanced equation. Now, when we're looking for the unknown, they may ask us to find the unknown in moles, atoms, ions, molecules, formula units, or grams itself. Remember, as we're going from an area where we know stuff because it's given to us, to an area where we know nothing at all, we have to make basically a leap from one side to the other side.
That's why we call this the jump as we go from the side where we are given information to the side where we have our unknown. Remember, when we make this jump, we have to do a mole-to-mole comparison. That's why a balanced equation is needed with stoichiometry because when we do this mole-to-mole comparison, we have to use the coefficients within the balanced equation. Knowing this basic setup for the stoichiometric chart will be essential for any type of titration, stoichiometric calculations that we're going to employ. Now that we've looked at that chart, come back, click on the following video and take a look at how I answer the following example question that's left on the bottom by using the stoichiometric chart and what we remember dealing with stoichiometry.